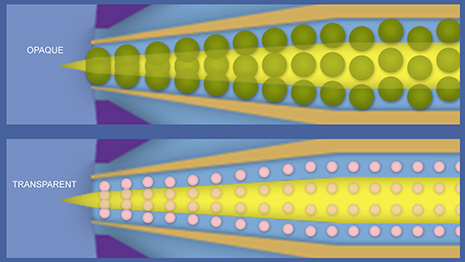
Pigments used in transparent paints have smaller particle size and are more widely dispersed than the pigments used for opaque paints. Particle size is of little consequence when brush painting but it can have a significant impact when the material is being forced through the small orifice of an airbrush. Very fine detail airbrushes used by artists and illustrators are designed for lightweight transparent materials such as watercolors and inks. Our model paints are generally opaque paints. And as you can see from these illustrations, just thinning an opaque paint will not completely overcome the negative flow characteristics of a larger particle size.
Paint for Scale Models

Any complete discussion of paints and paint chemistry would fill volumes. The varieties of proprietary mixtures are endless with each companies twist on a particular category of paint. The terminology applied to paints is often misused leading to a number of misconceptions about the different paint types. This results in a number of modelers turning to the practice of amateur anecdotal chemistry with sometimes mixed and inconsistent results. The following brief discussion is only meant to be helpful in defining terminology and explaining the basic characteristics of the most commonly used paints.
The main components of paint are pigments, binders, liquids, and additives.
Pigments are granular solids incorporated into the paint to add color, toughness, texture. Pigments can be classified as either natural or synthetic types. Natural pigments include various clays, calcium carbonate, mica, silicas, and talcs. Synthetics would include engineered molecules, calcined clays, blanc fixe, precipitated calcium carbonate, and synthetic pyrogenic silicas. Metallic paints use actual finely ground metallic particles. Hiding pigments make paint opaque and protect the substrate from the harmful effects of ultraviolet light. Hiding pigments include titanium dioxide, phthalo blue, red iron oxide, and many others. Alternatively, some paints contain dyes instead of or in combination with pigments. Some pigments are toxic, such as the lead pigments that are used in lead paint. Paint manufacturers began replacing white lead pigments with the less toxic substitute, titanium white (titanium dioxide), even before lead was functionally banned in paint for residential use in 1978 by the U.S. Consumer Product Safety Commission. The titanium dioxide used in most paints today is often coated with silica or alumina for various reasons such as better exterior durability, or better hiding opacity via better efficiency promoted by more optimal spacing within the paint film.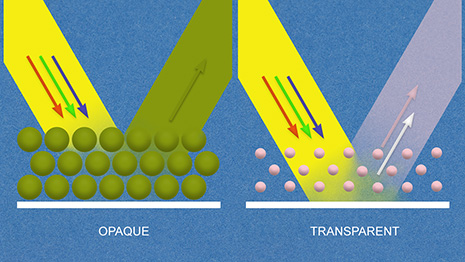
The binder is the actual film forming component of paint. It is the only component that must be present. Sometimes you will hear the term “vehicle” applied to the ingredients in paint, but this is an ambiguous term sometimes used to refer to the solvent or sometimes the binder. The binder imparts adhesion, binds the pigments together, and strongly influences such properties as gloss potential, exterior durability, flexibility, and toughness. Binders include synthetic or natural resins such as alkyds, acrylics, vinyl-acrylics, vinyl acetate/ethylene (VAE), polyurethanes, polyesters, melamine resins, epoxy, or oils.
Binders can be categorized according to drying, or curing mechanism. The four most common are simple solvent evaporation, oxidative cross linking, catalyzed/cross linked polymerization, and coalescence. There are also less common curing mechanisms. Drying and curing are two different processes. Drying generally refers to evaporation of the solvent or thinner. Curing refers to polymerization of the binder. Depending on the chemistry and composition, any particular paint may undergo either or both processes. So some paints only dry, some paints dry and then cure, and there are those that do not depend on drying for curing.
Paints that dry by simple solvent evaporation and contain a solid binder dissolved in a solvent are known as lacquers. The term lacquer originates from the Portuguese word for lacre, a type of resin excreted from certain insects. The use of lacquer paints can be traced back to ancient China, Japan, and India. These “lacquers” were derived from both insects as well as the T. vernicilflurrm or “varnish tree.” In modern usage, lac-based varnishes are referred to as shellac, while lacquer paint refers to other polymers dissolved in volatile organic compounds (VOCs), such as nitrocellulose, and later acrylic compounds dissolved in lacquer thinner. Lacquer thinner is a mixture of several solvents typically containing butyl acetate and xylene or toluene. The classic Floquil was a type of xylene based lacquer which is why it had a characteristic smell that differed from the typical lacquer thinner.
In a general sense, lacquer is a clear or colored varnish that dries by solvent evaporation, and sometimes a curing process as well, that produces a hard, durable finish in any sheen level from ultra matte to high gloss and that can be further polished as required. A solid film forms when the solvent evaporates, and because the film can re-dissolve in solvent, lacquers are not suitable for applications where chemical resistance is important. Classic nitrocellulose lacquers fall into this category. Quick-drying solvent-based lacquers that contain nitrocellulose, a resin obtained from the nitration of cotton and other cellulostic materials, were developed in the early 1920s, and extensively used in the automobile industry for 30 years. Prior to their introduction, mass produced automotive finishes were limited in color, with Japan Black being the fastest drying and thus most popular. General Motors Oakland automobile was the first (1923) to introduce one of the new fast drying nitrocellulose lacquers, a bright blue, produced by DuPont under their Duco trade name. These lacquers are also used on wooden products, furniture primarily, and on musical instruments and other objects. The nitrocellulose and other resins and plasticizers are dissolved in the solvent, and each coat of lacquer dissolves some of the previous coat. These lacquers were a huge improvement over earlier automobile and furniture finishes, both in ease of application, and in color retention.
The preferred method of applying quick-drying lacquers is by spraying, and the development of nitrocellulose lacquers led to the first extensive use of spray guns. Nitrocellulose lacquers produce a very hard, durable finish that can be polished to a high sheen. Performance varies by formulation, but lacquers generally tend to have better UV resistance and lower corrosion resistance than comparable systems that cure by polymerization or coalescence. Drawbacks of these lacquers include the hazardous nature of the solvent, which is flammable, volatile and toxic. Lacquer grade of soluble nitrocellulose is closely related to the more highly nitrated form which is used to make explosives. They are generally not toxic after about a month after the solvent has completely evaporated and any polymers have cured.
Lacquers using acrylic resin, a synthetic polymer, were developed in the 1950s. Acrylic resin is colorless, transparent thermoplastic, obtained by the polymerization of derivatives of acrylic acid. Acrylic is also used in enamel paints, which have the advantage of not needing to be buffed to obtain a shine. Enamels, however, are slow drying. The advantage of acrylic lacquer is its exceptionally fast drying time. The use of lacquers in automobile finishes was discontinued when tougher, more durable, weather and chemical resistant two-component polyurethane coatings were developed.
Due to health risks and environmental considerations involved in the use of solvent-based lacquers, much work has gone in to the development of water-based lacquers. These lacquers are considerably less toxic and more environmentally friendly, and in many cases, produce acceptable results. More and more water-based colored lacquers are replacing solvent-based clear and colored lacquers in the automobile and other similar industrial applications. Water based lacquers are used extensively in wood furniture finishing as well.
Paints that cure by oxidative cross linking are generally single stage coatings. When applied, the exposure to oxygen in the air starts a process that crosslinks and polymerizes the binder component. Classic alkyd enamels would fall into this category. These paints typically use a petroleum-based solvent which is why they are often referred to as oil-base or oil paint. Oil based paints were the first type of paint used in plastic modeling. Alkyd enamels, dry and cure from the inside to the outside. Sometimes this process can take weeks. Oxidative cure coatings are catalyzed by metal complex driers such as cobalt naphthenate.
The newest types of paint are acrylics. The origin of these paints can be traced back to the early work of Rohm and Haas. Otto Rohm and Otto Haas were German immigrants who formed a small Pennsylvania based company. Following WWI, they were able to concentrate on Rohm’s earlier acrylic polymerization work and develop prototypes of what was to become known as Plexiglas.
One of their biggest challenges was synthesizing acrylic monomers; the single molecule building blocks used to form polymers. Producing these monomers was expensive, time-consuming and generated relatively low yields. These difficulties limited potential markets, especially in areas of coatings and molding powders. Rohm would work on this problem until his death in 1939. Haas’s quest to develop an efficient and economical synthesis of acrylic monomers began in earnest in 1946 and would take three years.
In 1951, Haas authorized the construction of a new plant in Houston, Texas. The plant began producing acrylate monomers in 1952. The first paint emulsion, Rhoplex AC-33, appeared a year later. AC-33 was the critical binder of these new paint formulations. Rhoplex AC-33 and the other acrylic emulsions that Rohm and Haas would develop in the years ahead have some amazing qualities. Emulsion polymers are prepared in water and stabilized with surfactants, molecules that are hydrophilic (“water-loving”) in one segment and hydrophobic (“water-hating”) in the other. Emulsion polymers begin forming when a free radical, acting as an initiator, breaks a double bond between two carbon atoms in an acrylic monomer, starting a reaction that causes as many as 10,000 monomer units to bind together into a polymer chain. As these chains take shape, they grow into submicron-sized spheres. Within each sphere there are about 300 acrylic polymer chains.
As the spheres disperse in the solution, the water changes color: turning chalky white with a consistency similar to skim milk. These spheres are incredibly small. A 200-gram sample of an acrylic emulsion, about one-tenth the amount used in a typical gallon of paint, contains more of these submicron-sized spheres than there are stars in the Milky Way. The polymers created in the emulsion are easy to handle and formulate. But perhaps their most amazing characteristics are their extremely high molecular weights, which approach one million. Because of this, as the water evaporates the polymers coalesce into a tough acrylic film. These films, depending on how they are prepared, can have the tackiness of “fly paper” or the hardness of glass. In addition, for the creative chemist, emulsion polymers offer a number of “dials to turn” to optimize properties. These include backbone composition, molecular weight, functional monomers, morphology and surface chemistry.
Acrylic emulsions offer another important advantage: they’re environmentally friendly. As Rhoplex AC-33 and other acrylic emulsions supplanted solvent-based paint components, their water-based, non-toxic, non-flammable nature reduced harmful emissions and enhanced worker safety.
Acrylic binders are used in all forms of paints so that you see acrylic lacquers and acrylic enamels (petroleum based). These were the most common types of automobile paints in use until the introduction of the two-stage (color coat, clear coat) catalyzed paint systems. These acrylic paints used special solvents to reduce the acrylic binders. Anyone who has shot these acrylic formulations is familiar with the characteristic odor of the thinners. In modeling, our acrylic paints are generally water or alcohol based.
Paints that rely on catalyzed/cross linked polymerization are two part urethanes and epoxy type paints. They require that a catalyst be added to the paint. These types of paints are most commonly used in automotive finishes. They are highly toxic and should be used with care.
Latex paint is a water-borne dispersion of sub-micrometre polymer particles. The term "latex", in the context of paint, simply means an aqueous dispersion. Latex rubber (the sap of the rubber tree that has historically been called latex) is not an ingredient. These dispersions are prepared by emulsion polymerization. Latex paints cure by a process called coalescence where first the water, and then the trace, or coalescing, solvent, evaporate and draw together and soften the latex binder particles and fuse them together into irreversibly bound networked structures, so that the paint will not redissolve in the solvent water that originally carried it. The residual surfactants in latex paint as well as hydrolytic effects with some polymers cause the paint to remain susceptible to softening and, over time, degradation by water.
The liquids in a paint typically account for somewhere between 25 and 50 percent of the paint’s volume. Their function is to carry the pigments and the binders. There are two different types of liquids used in paint formulas: solvents and dilutents. Solvents hold the binder and pigment in suspension until the paint sets. In oil-based paint, the solvent is an organic material such as a paint thinner. In latex paint, water serves as the solvent. Dilutents also help to keep the pigments and binders in suspension, but they are used primarily to reduce the cost of the paint. High quality paints tend to have lower levels of dilutents.
In addition to these broad categories of paint, each paint company may use additives to improve or enhance certain qualities of their paints. So no paint is generic, and it is important to keep this in mind when mixing and thinning paints.
Paints For Model Building
Of course we are all free to paint our models with whatever we choose. Depending on the material that your model is constructed from, you might indeed want to use a paint that was not specifically formulated for model builders. In this regard, wood and metal come readily to mind. For modelers who are searching for high quality enamel paint, I would suggest looking into One Shot enamels that are the standard for sign painters.
When I was building 1/12th scale cars and motorcycles, I was almost always using urethane automobile paints and catalyzed clear coats. Single component urethanes also come formulated specifically for airbrush use and are quite popular with airbrush artists involved in the automotive field. Zero paints sold for model cars are single component urethanes. Caution should be exercised with the use of all urethanes as they are isocyanides and carry extreme health risks. Always use a respirator mask and avoid contact with skin.

As model builders, we will most commonly be using one of the many paints that have been developed specifically for our needs and, “developed specifically” is the key. Paint is all about chemistry. Each brand of paint has been created with specific pigments, binders and additives into a system to hopefully optimize their use. When you start mixing and thinning, you want to do it in a way that is consistent with the chemical system that you are using so that you do not adversely affect the qualities of the paint. Don’t be that duck who posts a photo of his or her 5 year project with a crazed or crackle finish. We’ve all seen these posts. It always starts with...”What went wrong?” This post might be followed by some even more entertaining suggestion, “I always thin my paint with BBQ lighter fluid. It gives me a smokin’ finish.”
When all else fails, you might want to give the manufacturer’s directions a try. Using the products made specifically for the thinning and over coating of your particular brand of paint are usually safe bets. Now that I’ve said this, is that what I always do? No. Is that what you are always going to do? Probably not. So acting on the premise that your mothers admonitions had about a 50% rate of effectiveness, it is probably worthwhile to look at how we can alternatively handle these materials with a degree of certainty so that we have reliable and stable results. The following are some general “practical” guidelines.
As a general rule, if you are using various categories of materials over each other, lacquer goes down first, followed by either aqueous acrylic or oil based enamel. Lacquer solvent applied over either aqueous acrylic or oil based enamel can yield some pretty nasty results, if not immediately, then on occasion over time.
I say “can”, because if you are careful to apply, in a more “dusting” fashion such as a light clear flat coat, you will probably get away with it. If however, you are laying on a heavy coat of yellow lacquer over your nice coat of dark green WEM ColorCoat, you might see some interesting results. Results also vary with the degree of drying or curing of the underlying layer. A coat of oil based enamel that has been sitting around all summer will be more resistant than one that was only applied recently.
Oil base paints such as Humbrol, Model Master, Xtracolor, are in some respects, more forgiving in the way they are thinned. They usually respond well to being thinned with good grades of either mineral spirits (paint thinner) or lacquer thinner. However, as mentioned above, too much lacquer thinner over oil based enamel can result in a problem. Just because you thinned your paint with lacquer thinner, you didn’t magically turn it into a lacquer. Remember, oil based enamels mainly cure.
Our modeling acrylics are available in forms of both solvent and aqueous based. Some of these can be compatible. In this case, I have to admit, any numbers of things seem to work or go wrong, so I would recommend that if you are having inconsistent results, fall back to the manufacturers guidelines. I found a particular post on the Missing-Lynx forum board by Pat Stansell, of Ampersand Publishing, to be extremely informative:
“Most modern paints (post WW2) are polymer based. Polymers are the base chemical elements for plastics. The term "acrylic" is indicative of that composition. Acrylic paint is essentially polymers suspended in a water-soluble base. There are always two components to paint: the pigment and the vehicle. In this case, the polymers are the pigment and the water-soluble solution is the vehicle. The advantages are that the vehicle is very easily discarded once the paint is applied (water evaporates), leaving only the polymers.
In modeling paints, these polymer particles are very fine, but also very susceptible to hardening. When even small amounts of air are introduced to the mixture, it begins to harden. The most common effect of this is the creation of very small bubbles. These form micro spheres of hardened paint that can increase the viscosity of the mixture. This is also why shaking acrylic bottles is not a great idea.
The use of lacquer thinners for acrylics is logical if we realize that as petroleum distillates, these thinners are sort of the "antidote" to polymers. They dissolve, rather than suspend them. This chemical reaction has the effect of eliminating the micro spheres that can impede the flow of paint inside your airbrush. Additionally, the viscosity of lacquer thinner is higher than that of water or isopropyl alcohol (just as isopropyl alcohol is higher than that of water).
Why doesn't Tamiya just tell you this? Well, acrylic modeling paint is a bit of a big deal because it is supposed to be mostly, or completely non-toxic. This is a big marketing tool for attracting children to the hobby. Having two paint lines and their respective thinners, one non-toxic and the other "not," makes selling them easier.
Also, storing acrylics in thinner would probably result in an increased degradation of the polymers, making it yukky (a technical term). By atomizing the paint in an airbrush, we speed the evaporation of the thinner and prevent any additional nasty breakdown of the paint.
Thinners such as LT, mineral spirts, varsol, etc are not pure chemicals but mixtures of a number of chemical. Thus one LT is not the same as the other. Most of the "paint store" LT is a bit more "aggressive". It is great for airbrush clean up but I would not take the chance using it to thin the paint. The Tamiya LT is the same product used in the Tamiya paint....what gives it the distinctive smell.
Pure chemicals (to some extent) are Isopropyl Alcohol (actually around 90% IPA in Water), Windex (3% Ammonia in water with detergent) and plain water. As far as evaporation rate, or how fast the paint will dry, LT is the fastest followed by IPA, Ammonia Water and then Water. Because of the surface tension effects of water, I would not recommend it. The Ammonia water with detergent works as the detergent counters the surface tension of water and the Ammonia speed up the evaporation (drying). Remember that windex is corrosive to some airbrush parts. IPA is an effective general purpose thinner for all acrylics. It tends to be a bit better with MM and Poly-Scale paints. LT is most effective with Tamiya and Gunze and IMO far superior to any other solvent.
DO NOT use mineral spirits, white spirits, Varsol or turponiod with any acrylic. These formulations are designed for oil/enamel based paints and may lead to a gloppy mess with acrylics.
I know....now I really confused everyone.”
I think I will let Pat’s last words end this discussion.
Airbrush Paints for Scale Modeling
Dyes and Watercolors
Dyes and Watercolors
Once watercolors are thinned with water, they have approximately the same flow rate in an airbrush as dyes. It is for this reason that many airbrush artist’s think of water colors and dyes as one and the same, except for perhaps the range of colors available in each medium. But there is an essential difference, because watercolors derive their color from pigments and dyes have no pigment.
Dyes derive their color from chemical substances (either natural or synthetic) that are dissolved in water. With a dye, water is the vehicle (or color carrier) and the means of dilution. Dyes color the surface they are applied to by staining it, so dyes have no binder, the substance that binds paint to a surface.
Watercolors, on the other hand, derive their color from pigment that has a binder as a vehicle and that uses water as the means of dilution. The vehicle is generally a solution of gum arabic (often with a plasticizer such as glycerin if it is a tube water color, not a cake or pan water color). The vehicle both carries the color and helps to bind the watercolor to the surface to which it is applied.
Another essential difference between dyes and watercolors is their permanence. Dye is transitory (sometimes referred to as "fugitive") color because all dyes fade when exposed to light over a period of days or weeks. Because watercolors derive their color from pigment crystals of natural or synthetic compounds suspended in a vehicle, they tend to be more permanent. Artists who wish to work in a very wet medium, and who are seeking permanency for their work, cannot work in dye but must choose watercolor instead. And to achieve the richest color one must choose a highly pigmented watercolor. The relative strengths of comparable watercolors can be experimentally determined by a standard volume trictest. Grumbacher's Thalo Crimson Finest brand watercolor and what is called a "student grade" watercolor of similar color value were diluted to a ratio of 1 to 30, color to water, and applied in a single application on #147 cold press Arches watercolor paper using a fine-spray, double action airbrush held 14 inches from the surface of the paper. After the two had dried the Thalo Crimson exhibited a stronger color and pigment load. Another important ingredient in watercolors is the vehicle. Like the pigment, the vehicle comes in various grades and only the best grades are used in making a quality watercolor. Also, like watercolor pigments, the purity and clarity of the watercolor vehicle are important ingredients to the subsequent color and intensity
Opaque Watercolor Gouache
For opaque effects, the airbrush artist can safely use artists' quality gouache. A well dispersed gouache has all the positive attributes of a fine watercolor, and when diluted properly it has the same flow rate as watercolor. The difference between gouache and transparent watercolor is that a fine chalk has been added for opacity without altering the color. Gouache offers the airbrush artist an enormous palette. Some lines have over 50 different colors. And while gouache is not generally considered an enduring or "archival" medium, many of the blues, greens and earth colors in Grumbacher's Designer's line of gouache, for example, are rated "Permanent" and "ExtremelyPermanent."